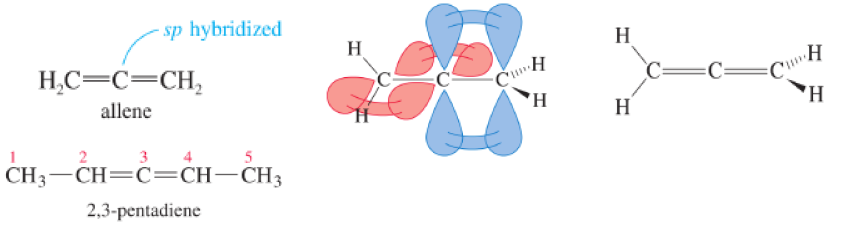
Carbon atom 3 is the sp hybrid allene-type carbon atom. Carbons 2 and 4 are both sp2 and planar, but their planes are perpendicular to each other, Each carbon must have 2 different substituents.
Geometrical isomers: Two stereoisomers (diastereomers) have the same molecular formula but differ in spatial arrangement due to one of 2 reasons:
1- Alkenes, there is restriction for the free rotation around double bond.
2- Cycloalkanes. there is restriction for the free rotation.
- The 2 stereoisomers (diastereomers) have different physical & chemical properties, While, Open-chain alkanes undergo rotations about their carbon–carbon single bonds.
Example:
Nomencalture of geometric isomers: The 2 groups around each carbon on the double bond ordered according to the priority rule.
If one carbon of the two carbons of the double bond has 2 identical substituents: no geometrical isomers
Geometrical isomers in cyclic compounds :-
● Cycloalkanes are alkanes that contain rings of carbon atoms, For example, the cycloalkane with four carbon atoms in a ring is called cyclobutane.
● Cycloalkanes are similar to alkenes, as there is restriction for the free rotation around the C-C bond.
● So cycloalkane has two distinct faces.
● If two substituents point toward the same face, they are cis.
● If they point toward opposite faces, they are trans.
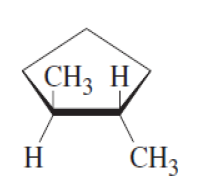 |
| trans-1,2-dimethylcyclopentane |